Product safety:
Ongoing research activities represent an important basis for fulfilling the safety aspects of Class IIa certified medical products. The chemical and physical characterisation of the specific PMA-Zeolite is fundamental. The pH stability to acids and bases as well as temperature stability and the appropriate particle shape (round and no needle-shaped particles) should be demonstrated. According to ISO standard, recording of safety entails tests for cytotoxicity, sensitisation, and irritation. In addition to the tests required by the MPG, toxicological studies (sub-acute, acute, and chronic) as well as tests for genotoxicity and reproductive/developmental toxicity must be performed in order to ensure additional safety. The safety-relevant aspects were examined and evaluated by both the designated notified body as well as experts in the field.
Thanks to these tests (including expert reports), the safety of PMA-Zeolite for human use and the reach of efficacy of the gastrointestinal tract were clearly defined.
Mechanism of action:
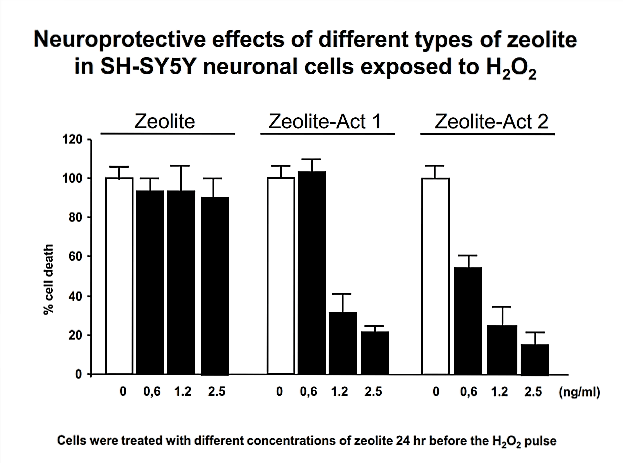
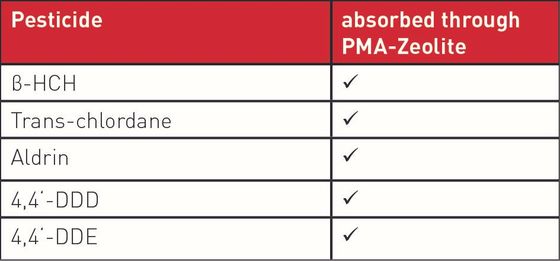
Based on the clear evidence for safety, the mechanism of action was defined. With regard to the recognised detoxifying potential of zeolite-clinoptilolite, as part of the basic research, the capacity of PMA-Zeolite with respect to environmental toxins (including the heavy metals lead, cadmium, arsenic, chromium, and nickel a well as the metabolic end product ammonia) was analysed in vitro using a gastrointestinal model. In this case, the ion exchange capability, and the resultant selective binding of defined pathogenic substances, was clearly proven. Details on the structure and mechanism of action of PMA-Zeolite can be found in the background information section.
Operative hypotheses and proof of efficacy:The defined main mechanism of action plays a fundamental role in demonstrating the mode of action of PMA-Zeolite at the site as well as validating various operative hypotheses based on this defined mechanism of action. The data, insights, and trends from preclinical studies (in vitro and in vivo) – especially clinical gold standard studies – form the basis for further validation (human applications).
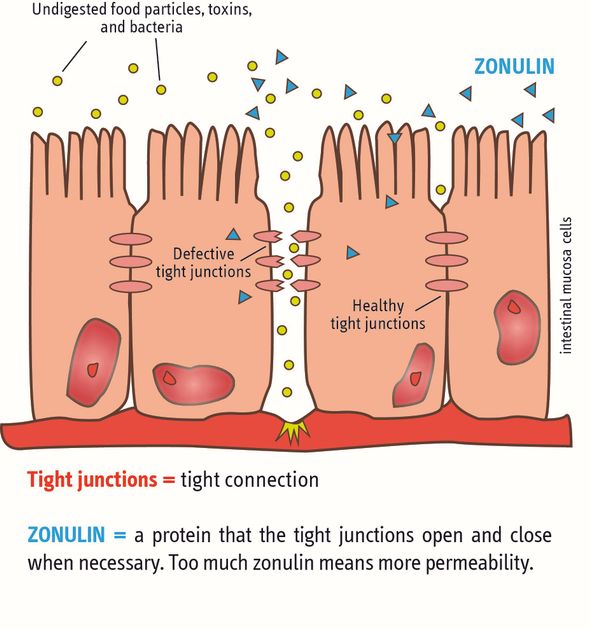
Evidence-based proof of the intended main effect:One hypothesis based on the main mechanism of action (selective binding of pollutants in vitro gastrointestinal model) is the strengthening of the functionality of the intestinal wall barrier. This promising hypothesis was proven in a randomised placebo-controlled clinical gold standard study in which a significant decrease in the bio-marker zonulin (and thus an improvement/strengthening of intestinal wall function/integrity) was achieved. This represents the main intended action of PMA-Zeolite.